A recent decision by the Patent Trial and Appeals Board (“Board”), in Ex Parte Manetch, 2022 Pat. App. LEXIS 1531, emphasizes the need for hard evidence to support the patentability of a prodrug of a known molecule. The application at issue, U.S.S.N. 16/072,088, included claims to a carbonate prodrug of ELQ-300. The prodrug has the following chemical structure, with the prodrug portion circled:
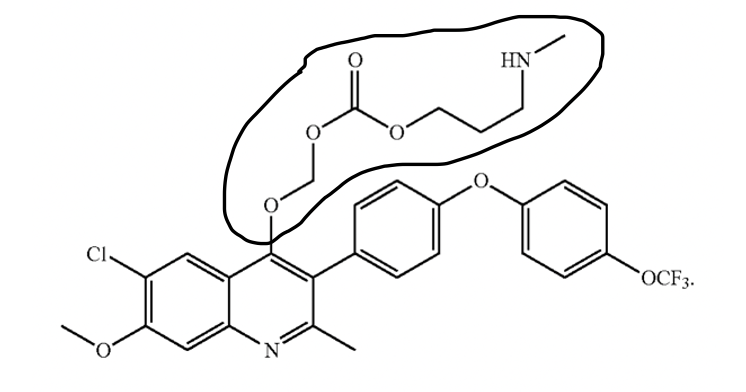
ELQ-300 is an antimalarial drug that entered preclinical development in 2013 but failed to progress further due to low aqueous solubility and poor oral bioavailability.
In his rejection, the Examiner applied the lead compound analysis described in Bristol-Myers Squibb Co. v. Teva Pharmaceuticals USA, Inc., 752 F.3d 967, 973 (Fed. Cir. 2014) to find the prodrug obvious, citing two references in support of his prima facie case of obviousness. The first reference, US 2012/0115904 A1 (“Riscoe ‘904”), taught that ELQ-300 has strong activity against malaria, but that it could not be considered for further pharmaceutical development because it lacked aqueous solubility. The second reference, a 2014 Ph.D. dissertation by Monastyrskyi and Andrii (“Monastyrskyri”), taught that the solubility issues associated with structurally similar antimalarial compounds could be overcome by modifying the compounds with a carbonate prodrug – the same carbonate prodrug that Manetch selected for ELQ-300.
The Applicant did not respond with any unexpected results. Instead, the Applicant simply argued that the prior art did not adequately disclose how one would synthesize the ELQ-300 prodrug.
According to the Applicant,
"changes in the lead compound isolation protocol, the reaction concentrations, the reactiontemperature, the reaction time, the equivalents of reagents used, all had to be modified in order to successfully produce the compounds of claims 4 and 5. The level of optimization undertaken amounts to more than undue experimentation"
This was not enough to overcome the Examiner’s prima facie case of obviousness. According to the Board, the Applicant had used the same synthetic scheme used in the prior art with similar anti-malarial drugs. The Applicant had to optimize some of the reaction conditions, but this was merely routine optimization. As explained by the Board:
“We note that while the Manetsch Declaration recites that optimization was required, the Manetsch Declaration does not assert that the particular synthetic approach required more than optimization and does not indicate that anything other than routine optimization of the reaction conditions and isolation conditions was performed. Therefore, while we give the Manetsch Declaration some weight, we do not find it overcomes the Examiner's strong prima facie case of obviousness.”
The case highlights the need for unexpected results to overcome an examiner’s prima facie case of obviousness in the prodrug context, particularly when the problems with the parent molecule are in the prior art, and the use of a prodrug to resolve those problems is also known.
