In Ex parte CHENGZHI ZHANG, 2019 PAT. APP. LEXIS 9373 (PTAB 2019), the Patent Trial and Appeals Board reversed the Examiner's final rejection of claims directed to a deuterated form of nilotinib, based on the position of the deuterium substitution, and the unpredictability of results obtained from deuteration at any specific position.
Deuteration of pharmaceutical compounds is not new, and is primarily undertaken to improve the metabolic fate of the compound. One of the references cited by the Examiner in this case, the "Gant '555 patent," described several compounds that had been successfully deuterated to:
"increas[e] the half-life (T[1/2]), lower[] the maximum plasma concentration (C [max]) of the minimum efficacious dose (MED), lower[] the efficacious dose and thus decreas[e] the non-mechanism-related toxicity, and/or lower[] the probability of drug-drug interactions."
Ex parte Zhang involved the deuteration of nilotinib, a compund which, the inventors admitted made a good candidate for deuteration because it "undergoes extensive oxidative metabolism via CYP3A4, and to a lesser degree by CYP2C8, CYP2C9, and CYP2D6". These cytochrome enzymes "react with and convert [] foreign substances to more polar intermediates or metabolites for renal excretion. Such metabolic reactions frequently involve the oxidation of a carbon-hydrogen (C-H) bond."
Given these known metabolic problems with nilotinib, and the known ability of deuteration to solve such problems with other compounds, the examiner had rejected Zhang's patent claim as "prima facie obvious per se."
On appeal, Zhang focused on the particular site of deuteration recited in its claims. The claim on appeal is reproduced below, where one can see that Zhang was not attempting to claim all deuterated forms of nilotinib, but was oinly attempting to claim nilotinib deuterated at one specific position.
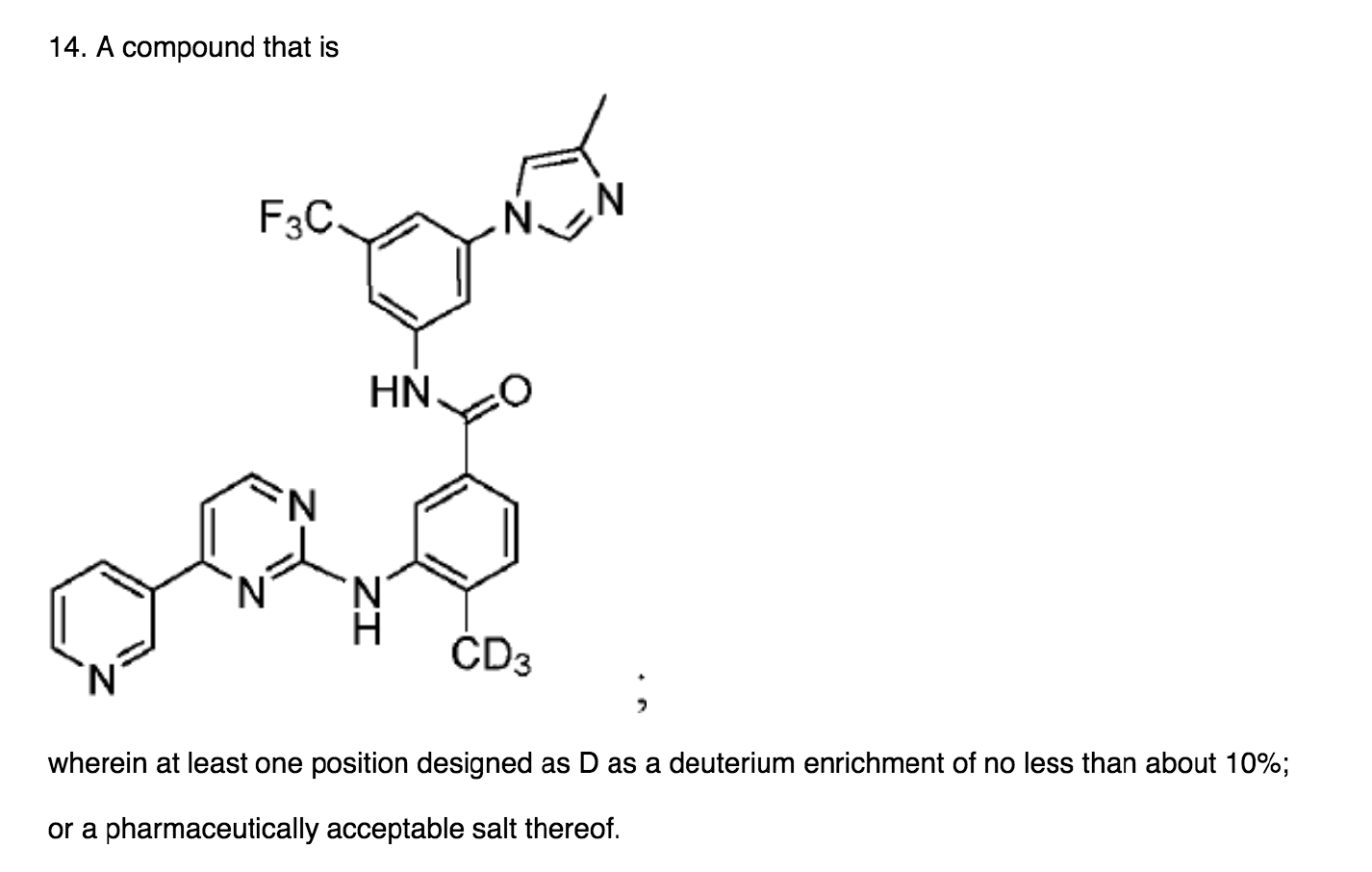
The PTAB found:
If we apply the lead compound analysis, we might agree with the Examiner that nilotinib is a reasonable lead compound based on the teachings of Manley and Breitenstein and that Gant provides several generic reasons to modify nilotinib by deuteration. However, claim 14 is not a generic modification of nilotinib by deuteration but rather is a modification of a particular position on the molecule. The Examiner provides no reason to make the particular modification required.
Continuing, the PTAB stated:
Appellant has provided a number of disclosures demonstrating that deuteration of different chemicals yields unpredictable results. ... Gant '555 at best provides general guidance that deuteration of molecules may improve their properties. While deuteration might represent a promising field of experimentation, Gant '555 itself demonstrates that the results of deuteration are not predictable. There is also no persuasive evidence that the experimentation required to identify a deuterated nilotinib would have been merely routine as asserted by the Examiner.
Because claim 14 covered a specific deuteratized position on nilotinib, and deuteration generally yields unpredictable results, the PTAB concluded that claim 14 was not obvious and reversed the Examiner's final rejection.
